Energetic Analysis of Shock Wave Formation and Loss Mechanisms during Spark Ignition of NH₃/H₂ Mixtures
- type:Bachelor- / Masterthesis
- time:arranged in consultation
- place:
- Zusatzfeld:
Content
Ammonia is a carbon-free energy carrier (emitting no CO₂) and possesses a high volumetric energy density compared to hydrogen (H₂). A significant advantage lies in its handling: NH₃ is relatively easy to store and transport, and the necessary infrastructure is largely already in place. However, the low reactivity of NH₃ compared to hydrocarbons complicates successful and stable ignition. This challenge is overcome by mixing NH₃ with H₂. Since H₂ is a highly reactive fuel, the ignitability and combustion behavior of the entire mixture are significantly improved.
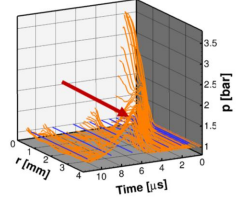
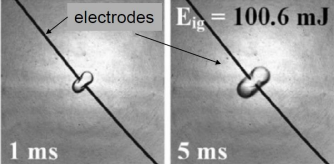
If an ammonia-hydrogen-air mixture is ignited by a spark with a short duration (< 10 µs), the rapid local heat input creates a pressure wave that influences the ignition process. Due to this pressure wave, successful ignition requires higher energy because the wave transports a portion of the input energy away from the ignition volume. This drop in temperature, or "ignition energy loss“, caused by the shock wave is to be investigated within the scope of this thesis.
Scope of Work
- Familiarization: Literature review regarding the numerical investigation of ignition processes, specifically considering pressure waves in ammonia-hydrogen mixtures.
- Simulation: Execution of simulations to determine the minimum ignition energy required, comparing scenarios with and without pressure wave consideration at short ignition durations.
- Energetic Analysis: Development of an evaluation method to quantify the energy contained in the propagating pressure wave and distinguish it from the thermal energy input.
- Evaluation: Quantification of ignition efficiency. Specifically: What proportion of the spark energy introduced into the gas is actually available for heating the flame kernel? How does this availability change as the spark duration decreases?