Multi-functional conversion of chemical species and energy
- contact:
- project group:
GM1 und MM1
- funding:
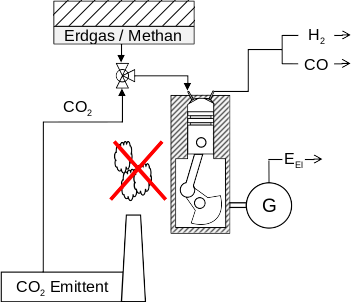
Reduction of CO2 exhaust gases
Prinzip & Idee:
- At high temperatures, CO2 and CH4 react to form synthesis gas:
CO2 + CH4 CO + 2 H2 - Temperature increase by adding noble gases and parallel exothermic reaction.
- CO2 is converted into important raw materials.
Application:
- Exhaust gas after treatment
- Chemical industry
Challenges and research areas:
- Feasibility study (experimental and numeric)
- Optimization of target variables:
- CO2 conversion
- Synthesis gas quality (CO/H2 ratio)
- Thermal efficiency
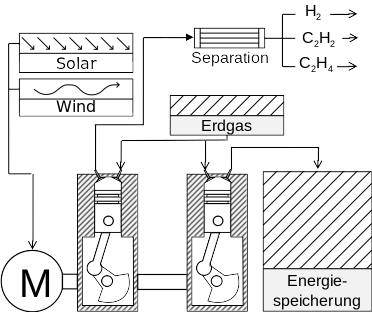
Pyrolysis of natural gas
Principle & Idea:
- Natural gas is only heated by compression (T = 1500 - 1800 K)
- No addition of oxygen Endothermic Reaction
- Natural gas react to form more valuable species (H2, C2H2, C2H4)
Application:
- Energy storage
- Chemical Reactor (extraction of valuable species)
- Use of combustible exhaust gases (ex: steel furnaces)
Challenges and research areas:
- Experimental investigation
- Underlying Chemistry ( ex: 2 CH4 3 H2 + C2H2 )
- Optimal operating points
- Maximum yields, conversions of natural gases